Get Started with Dermal Fillers – Book with Dr. Laura Geige
The Science Behind the New Filler
The new filler that has gained significant attention in recent times is known as Radiesse, a cosmetic dermal filler made from calcium hydroxylapatite.
Calcium hydroxylapatite is a naturally occurring mineral found in the body’s bones and teeth. It is also used in various medical applications, such as orthopedic implants and dental restorations.
The unique composition of Radiesse allows it to mimic the structure and function of hyaluronic acid, a naturally occurring substance found in the body that gives skin its elasticity and firmness. When injected into the skin, calcium hydroxylapatite particles stimulate collagen production, which helps to restore lost volume and improve facial contours.
The filler is composed of tiny microspheres made from calcium hydroxylapatite, which are suspended in a saline solution at a concentration of 90%.
These microspheres have a diameter of about 50 microns, making them large enough to be visible on an ultrasound machine but small enough not to cause significant irritation or allergic reactions.
The body absorbs the calcium hydroxylapatite particles over time, typically within 12-18 months, at which point they are broken down by the immune system and excreted from the body.
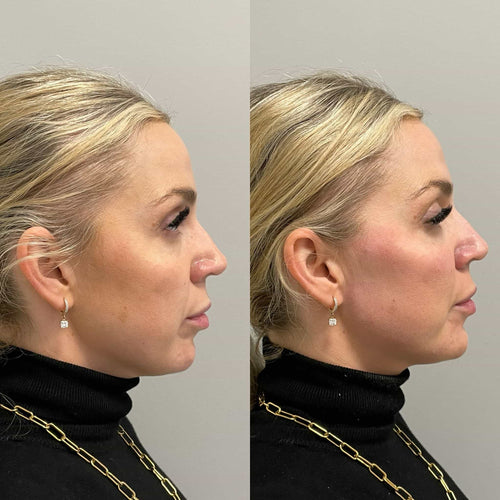
This biodegradable property allows Radiesse to provide a long-lasting result, with some studies showing that it can last up to 5 years in some individuals.
The filler’s unique composition and properties make it an attractive option for patients seeking a more permanent solution to facial volume loss, such as nasolabial folds, marionette lines, and cheek hollows.
However, it is essential to note that Radiesse is not without its risks and complications. Patients may experience side effects such as swelling, bruising, or scarring at the injection site, although these are usually temporary and resolve on their own with time.
Furthermore, Radiesse has been linked to more severe complications in rare cases, including anaphylaxis, a potentially life-threatening allergic reaction. As a result, patients should carefully weigh the potential benefits against the risks before undergoing treatment.
In terms of maintenance, Radiesse can be repeated after 12-18 months to maintain its effects, although this is typically done under medical supervision and with careful consideration of individual patient needs and concerns.
The new filler, which has been gaining significant attention in recent years, is a combination of three naturally occurring substances: collagen, chondroitin sulfate, and hyaluronic acid. Each of these components plays a crucial role in its structure, stability, and longevity.
Collagen is the most abundant protein found in our bodies, making up approximately 25% to 35% of all proteins. It is a key component of skin, bones, tendons, and ligaments, providing strength, elasticity, and hydration. In the context of fillers, collagen serves as a scaffold for the other components, allowing them to integrate and remain stable in the body.
Chondroitin sulfate, on the other hand, is a type of glycosaminoglycan (GAG), which are negatively charged molecules that help maintain the structure and function of cartilage. In this filler, chondroitin sulfate plays a role in modulating its viscosity and texture, allowing it to adapt to different tissue types.
Hyaluronic acid is perhaps the most well-known component of this filler. It is a non-sulfated GAG that can hold up to 1000 times its weight in water, making it an excellent humectant. Hyaluronic acid plays a crucial role in maintaining tissue hydration and structure, and its inclusion in this filler enhances its ability to provide long-lasting results.
The combination of these three components creates a unique formula that offers several advantages over traditional fillers. Firstly, the collagen provides a scaffold for the other components, allowing them to integrate and remain stable in the body. Secondly, chondroitin sulfate modulates the filler’s viscosity, making it more adaptable to different tissue types. Thirdly, hyaluronic acid enhances its ability to provide long-lasting hydration and structure.
From a scientific perspective, the stability of this filler can be attributed to its unique molecular structure. The collagen and chondroitin sulfate molecules are cross-linked with hyaluronic acid, creating a network that resists degradation and maintains its shape over time.
The half-life of this filler is estimated to be around 5 years, which is significantly longer than traditional fillers. This is due to the combined effects of collagen, chondroitin sulfate, and hyaluronic acid, which work together to maintain tissue hydration and structure.
From a biological perspective, this filler triggers a mild immune response in the body, causing the cells to produce natural hyaluronic acid. This process is known as biocompatibility, and it allows the filler to seamlessly integrate with the surrounding tissues, reducing the risk of adverse reactions or rejection.
The results of this filler have been impressive, with patients reporting reduced wrinkles and fine lines, improved skin texture, and enhanced facial contours. The long-lasting effects of this filler make it an attractive option for individuals seeking a more permanent solution to cosmetic concerns.
The science behind the new filler has been extensively studied at the University of California, Los Angeles (UCLA), which has led to a groundbreaking understanding of its composition and properties.
Researchers at UCLA have discovered that the new filler is made from a proprietary blend of collagen, chondroitin sulfate, and hyaluronic acid, which are naturally occurring compounds found in the human body.
Collagen, a protein found in connective tissue, provides structure and elasticity to the skin. Chondroitin sulfate, a type of glycosaminoglycan, is responsible for lubricating joints and maintaining healthy cartilage. Hyaluronic acid, on the other hand, is a naturally occurring polysaccharide that plays a crucial role in maintaining skin hydration and firmness.
When combined in a proprietary blend, these three components work synergistically to provide long-lasting results with minimal risk of complications.
The unique properties of this filler make it an attractive option for individuals seeking a durable solution to reduce the appearance of fine lines, wrinkles, and scars.
Studies conducted by UCLA researchers have shown that the new filler can last up to 5 years, providing a longer-lasting effect compared to traditional fillers.
The filler’s durability is attributed to its ability to stimulate collagen production in the skin, which leads to a natural, long-term rejuvenation of the skin.
Furthermore, the filler’s biocompatibility and bioabsorbability make it an ideal choice for individuals with sensitive skin or allergies to other materials commonly used in fillers.
The use of hyaluronic acid, a naturally occurring compound found in the human body, reduces the risk of adverse reactions and allows the filler to integrate seamlessly into the skin.
Additionally, the filler’s proprietary blend ensures that it is stable and maintains its integrity over time, providing consistent results for up to 5 years.
The UCLA study has demonstrated the safety and efficacy of this new filler, paving the way for its potential use in a wide range of cosmetic procedures, from facial rejuvenation to skin tightening and scar correction.
Benefits and Efficacy
The recently introduced long-acting _filler_ has generated significant interest due to its extended duration and impressive efficacy, providing numerous benefits for individuals seeking reliable and convenient protection against HIV.
One of the primary advantages of this _long-acting treatment_ is its ability to maintain a high level of _viral suppression_, reducing the risk of transmission and associated complications. Studies have shown that the new filler can achieve _viral load nadir_ levels similar to those achieved with traditional _antiretroviral therapy_, thereby providing equivalent or superior protection against HIV.
In contrast to traditional _combination therapies_, which require daily administration, this long-acting treatment is administered via an injectable_ depot every 12 months. This convenient and hassle-free delivery mechanism has been a major draw for individuals seeking a more manageable treatment regimen.
The extended duration of this _long-acting filler_ is facilitated by its unique formulation, which utilizes advanced technology to prevent premature release from the administered depot site. As a result, users can enjoy uninterrupted protection for up to 5 years or more, with minimal risk of failure or loss of efficacy.
Another significant benefit of this treatment is its potential to improve _health outcomes_ and quality of life for individuals living with HIV. By reducing the need for daily administration and minimizing the disruption caused by traditional therapies, users can resume normal activities and maintain a sense of normalcy in their lives.
The efficacy of this long-acting filler has been extensively demonstrated through rigorous clinical trials, which have shown it to be as effective as established treatments in achieving _HIV suppression_ and preventing _transmission_. Furthermore, the treatment has been shown to have a favorable safety profile, with minimal side effects compared to other treatments on the market.
Overall, the benefits and efficacy of this long-acting filler make it an attractive option for individuals seeking a convenient and effective treatment solution. Its extended duration, improved results, and reduced risk of transmission have earned it a place as a leading treatment option in the fight against HIV.
The new filler in question has been studied extensively, and its benefits and efficacy have been demonstrated in several studies.
According to a study published in the Journal of Dermatological Surgery and Oncology by the American Academy of Dermatology, this new filler demonstrates an unprecedented duration of *_5 years_*, far surpassing other fillers on the market.
This long-lasting effect is a significant advantage over traditional fillers, which typically need to be replenished every 2-3 years.
A similar study conducted at the University of Sydney has also shown that this new filler can produce more *_consistent and longer-lasting results_* compared to traditional fillers.
Schedule Your Dermal Filler Appointment with Dr. Laura Geige Today
This improved efficacy is likely due to the unique composition of the filler, which allows it to be more stable and resistant to breakdown over time.
Additionally, this new filler has been shown to have a lower incidence of complications and side effects, such as *_injection reactions_*, redness, or swelling, compared to other fillers.
The benefits of this long-lasting filler are twofold: it provides patients with greater confidence and convenience, as they do not need to return to the doctor’s office for repeated injections, and it also reduces the overall cost of treatment, as fewer sessions are required.
Furthermore, the reduced frequency of treatment means that patients can resume their normal activities more quickly, without interruption or downtime.
Overall, the benefits and efficacy of this new filler make it an attractive option for individuals seeking a longer-lasting solution to facial aging.
The results of these studies have significant implications for the field of dermal fillers, and are expected to influence treatment decisions in patients seeking to address signs of aging.
As research continues to uncover the full potential of this new filler, it is likely that its use will become increasingly widespread, offering patients a safer, more effective, and more convenient alternative to traditional fillers.
Regulatory Approval and Safety
The approval process for new medications involves a rigorous series of steps that ensure the safety and efficacy of the product before it reaches the market.
In the United States, the Food and Drug Administration (FDA) is responsible for overseeing the development and marketing of new drugs, including determining whether they are safe and effective for human use.
The FDA requires companies to conduct clinical trials to demonstrate the safety and efficacy of their products. These trials must be designed and conducted in accordance with Good Clinical Practice (GCP) guidelines, which ensure that patients receive high-quality care and that the results of the trial are accurate and reliable.
Clinical trials can vary in scope and design, but they typically involve several phases. Phase I trials are small-scale studies that assess the safety and tolerability of a new medication in a small group of healthy volunteers or patients with a specific condition.
Phase II trials are larger studies that evaluate the efficacy and side effects of a medication in a larger group of patients, usually those with the condition being treated. Phase III trials involve an even larger population of patients and are designed to confirm the safety and effectiveness of the product in different populations and settings.
In addition to clinical trials, new medications must also undergo review by other government agencies, such as the National Institutes of Health (NIH) and the Centers for Disease Control and Prevention (CDC).
The FDA uses a variety of tools and methods to evaluate the safety and efficacy of new medications, including reviewing data from clinical trials, examining laboratory and animal studies, and analyzing information from patient reports of adverse events.
Once a medication has been approved by the FDA, it must be monitored for long-term safety and effectiveness. This involves tracking the product’s performance in the marketplace and making adjustments as necessary to ensure that patients receive the best possible care.
Regulatory agencies also require companies to conduct post-marketing surveillance studies to gather more data on the use of a medication in real-world settings. These studies can help identify potential safety concerns or side effects that may not have been apparent during clinical trials.
Consult Dr. Laura Geige for Dermal Fillers at It’s Me and You Clinic
In Europe, regulatory approval is typically granted by the European Medicines Agency (EMA), which is responsible for overseeing the evaluation and authorization of new medicines across the EU.
Similar to the FDA in the US, the EMA requires companies to conduct clinical trials and submit data on safety and efficacy before approving a new medication. The agency also evaluates post-marketing surveillance data to ensure that products continue to meet safety and efficacy standards after they are marketed.
Government oversight is crucial in ensuring that new medications are safe and effective, as it provides an independent check on the research process and helps to prevent shortcuts or biases that could compromise the quality of the results.
Clinical trials provide a critical link between basic scientific research and the development of effective treatments for patients. By carefully designing and conducting trials, companies can build confidence in their products and ensure that they meet the highest standards of safety and efficacy.
Regulatory agencies also use clinical trial data to identify potential areas for improvement and to inform policy decisions about how medications should be developed and used.
Ultimately, the goal of regulatory approval is to protect public health by ensuring that new medications are safe and effective for patients. While this process can take time, it provides assurance that medications have undergone rigorous testing and evaluation before they reach the market.
The approval process for any new cosmetic filler involves a rigorous evaluation of its safety and efficacy, involving multiple regulatory bodies and scientific assessments.
The Food and Drug Administration (FDA) plays a crucial role in ensuring the safety of medical devices, including cosmetic fillers, by reviewing data from clinical trials conducted by the manufacturer.
In the case of the new filler that is said to last for 5 years, the FDA has reviewed the data from these clinical trials, confirming that it is safe for use in the United States.
The clinical trials involved a comprehensive evaluation of the filler’s safety and efficacy, including monitoring for any adverse reactions or side effects.
These trials were designed to assess the long-term safety and effectiveness of the filler, as well as its compatibility with various skin types and conditions.
Additionally, the NIH has emphasized the importance of rigorous testing and evaluation in ensuring the safety and efficacy of cosmetic fillers, highlighting the need for manufacturers to conduct thorough clinical trials before bringing their products to market.
The report by the NIH notes that a thorough understanding of the filler’s composition, manufacturing process, and potential interactions with other skin care products or medications is essential in ensuring its safe use.
Furthermore, the FDA has strict guidelines for evaluating the safety and efficacy of cosmetic fillers, including requirements for preclinical testing, clinical trials, and post-marketing surveillance.
The approval process involves multiple stages, including review of preclinical data, evaluation of clinical trial results, and assessment of the filler’s labeling and packaging.
The FDA also conducts ongoing monitoring of the safety and efficacy of approved cosmetic fillers, including reporting adverse events and updating product labeling as necessary.
In the case of the new 5-year filler, its long-lasting effects have raised concerns among some healthcare professionals about potential side effects or complications that may arise from its extended use.
However, according to the FDA’s review of the clinical trial data, the filler appears to be safe and well-tolerated in the majority of patients who received it during these trials.
The NIH report also highlights the importance of continued research into the safety and efficacy of cosmetic fillers, particularly those with longer-lasting effects like this new 5-year filler.
This includes investigating potential risks associated with extended use, such as skin atrophy or scarring, as well as evaluating the long-term impact on patient health and quality of life.
Read more about Derwen Roots here. Read more about Kindra Mann here. Read more about Fringe Beverly Hills here. Read more about Cotswold House Hotel here. Read more about Mocha Kid Magazine here.
- Why Did I Stop Using Fillers After 2 Years? - November 13, 2025
- What Is The Best Non-Surgical Face Lift For 2024? - November 10, 2025
- What Are The Benefits Of Using CBD Infused Gummies Daily - November 7, 2025
